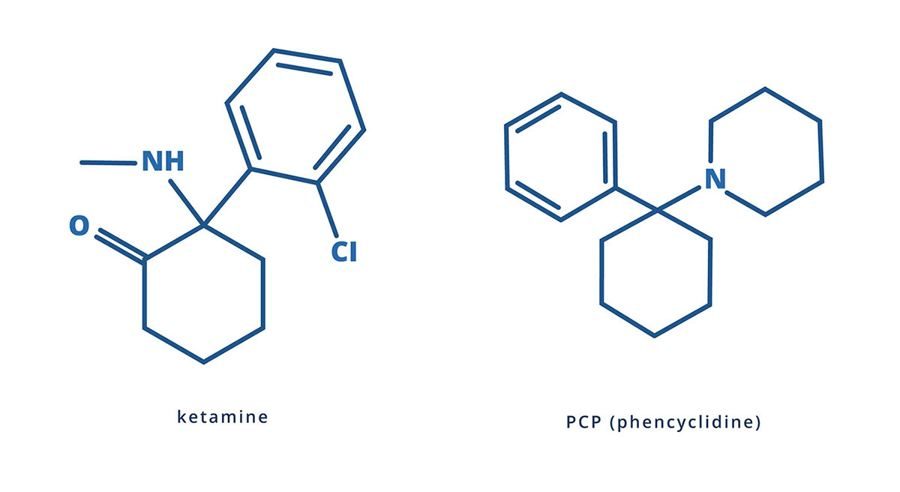
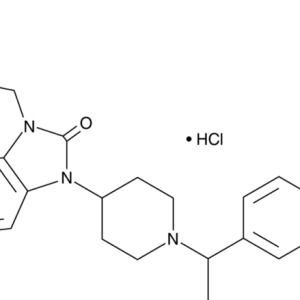
Phencyclidine (PCP) was synthesized by Parke Davis Co. (USA) and used as a general anesthetic in humans in the last half of the 50s of this century. It was distributed under the name “Sernyl”.
The use of PCP put a person into excellent surgical anesthesia without respiratory and cardiovascular depression. Unexpectedly for doctors, in 1959, data was obtained that a small percentage of patients (from 10 to 20%) to whom phencyclidine anesthesia was applied fell into a delusional state and experienced a hallucinogen-like effect. The presence of such a side effect led to the fact that PCR was withdrawn from legal circulation in the USA in 1965. In the early 60s, numerous laboratory studies showed that this drug is an effective anesthetic and immobilizer for various animals. In 1967, PCR began to be distributed in the United States under the name CERNILAN, as a veterinary anesthetic, mainly for primates. The use of “Sernilan” was discontinued in 1979.
In the early 60s, Parke Davis Co. (USA) synthesized and patented several phenycyclidine analogues, including PCE, PCPa, and TSP. The company tried to sell PC and TCP for use as surgical anesthetic products, but abandoned its idea.
Further chemical research led to Ketamine, which was first synthesized at the University of Michigan in 1965. Ketamine in therapeutic doses did not cause psychotic reactions. The Parke Davis company began its mass production (CI-581, Ketalar) as a safe general anesthetic with rapid onset of anesthesia and rapid withdrawal from it. This drug was widely used in Vietnam by the US Army.
PCP first appeared in illegal traffic in California in 1967. At that time, it was distributed in the form of capsules or tablets under the names “Peace Pill” and “Hog” (“Peace Pill” and “Capture”). The frequent use of high doses of the drug has led to extremely undesirable effects and consequences for the users. Information among drug addicts about this formed the basis for the decline in popularity of this drug. A few years later, however, PCP reappeared on the streets, initially as a solid form such as LSD, THC, or mescaline, or as a diluent of other substances. Drug addicts began mixing PCP powder or crystals with various plant materials, such as marijuana, tobacco, parsley seeds or mint leaves. These products were used for smoking. They were called “angel dust” and by the early 1970s had spread rapidly across U.S. cities. The peak of PCP abuse in the USA was observed in the late 70s and early 80s. By the early 90s, its consumption had dropped to a negligible level.
Analogues of PCP have also been found in illegal circulation in the United States. RCE was first discovered in California in 1979. It was distributed in the form of brown, purple, pink or cream colored powder or tablets. This drug was sold as PCP under the name “rocket fuel”. TSR and RSRu were first discovered in the early 70s. Both analogues were distributed under the guise of PCP in the form of powder or tablets. Around 1981, the use of phencyclidine analogues rapidly declined. To date, their use has been detected sporadically. In 1988, the appearance of TSRu in powder form was noted.
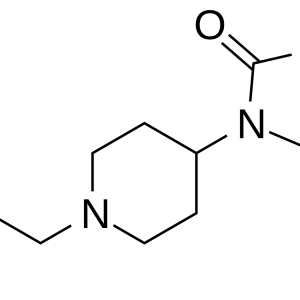
A similar situation is observed everywhere with regard to ketamine. It and its dosage forms have become widespread in the West and in our country under slang names: K, Ket, Ketamine, Special K, “Vitamin K”.
The total number of substances of this chemical class that have ever been synthesized and tested by pharmaceutical companies or seized from their illicit trafficking is, according to the DEA of the US Treasury Department, 5-6 dozen structures.

Pharmacology and toxicology of Arylcyclohexylamines
There are reports in the scientific literature describing the pharmacological effects of psychoactive doses of PCP in volunteers and surgical patients. A low single intravenous dose of 0.075 – 0.1 mg/kg of PCP causes alienation, through disorientation, a feeling of changing the shape of objects, negativism, drowsiness, apathy and a feeling of intoxication. High doses cause analgesia, dizziness, strange and unusual physical feelings, and disorientation. The ability to concentrate, learn, and memorize is deteriorating. At higher doses (1 mg/kg), anesthesia is observed, turning into catatonia and muscular rigidity. A small percentage of people develop side effects after coming out of analgesia, manifested in agitation, violence, convulsions, diplopia (double vision), dizziness and nystagmus (eye tremor).
A limited number of clinical studies have shown that TSP and RCE produce effects similar to PCP. At low doses, these analogues cause general immobilization, a feeling of “flying away” or “drifting” or severe intoxication. At higher doses, analgesia and anesthesia are associated with catatonia and general rigidity. During emergency withdrawal from anesthesia, some people experience intense agitation, violence, or hallucinations.
In laboratory animals, PCP and its analogues cause a wide range of pharmacological effects, including anticonvulsant effects, analgesia, catatonia, anesthesia, and ataxia. The exact mechanisms leading to the manifestation of such effects are not known. Animal studies have shown that PCR alters the functioning of various transmitter systems, including noradrenergic, serotonergic, and cholinergic. In addition, PCR alters the physiological metabolic processes of gamma-aminobutyric acid and some amino acids involved in nerve signal transmission. Many of these effects are due to the activation of probably existing PCP receptors. PCP analogues also act on these receptors.
The pharmacological properties of ketamine are used primarily for surgical anesthesia (anesthesia), including in veterinary practice. Anesthesia with the introduction of this drug has been called “dissociative”, which means, in particular, that consciousness is “separated” (or “dissociated”) during it from the body. Its main advantage over other anesthesia products is that it does not suppress laryngeal and pharyngeal reflexes (so you do not risk choking on your own saliva during surgery). In addition, Ketamine depresses breathing much less than other general anesthetics, and it even stimulates the heartbeat. In this regard, it is especially useful in emergency surgery (for example, in natural disasters, etc.), when dangerous side effects are especially undesirable.
The minimum effective anesthetic dose of ketamine for intravenous administration is 0.5 mg / kg of body weight, while consciousness turns off after 1 hour..2 minutes and the effect lasts about two minutes. At a dose of 1 mg / kg, anesthesia lasts about 6 minutes, and at a dose of 2 mg / kg – about 10-15 minutes. With intramuscular administration, the effect is slower, but more prolonged (with administration of 6..8 mg / kg, the effect develops after 6..10 minutes and lasts up to 30..40 minutes).
The analgesic (analgesic) effect develops within 10 minutes after intravenous administration and lasts approximately 2 hours..3 hours. With intramuscular administration, this effect is also more prolonged.
Ketamine is used for mononarcosis and combined anesthesia, especially in patients with low blood pressure or if it is necessary to maintain self-ventilation. It is indicated in emergency surgery and at the stages of evacuation, in particular in patients with traumatic shock and blood loss (due to rapid anesthesia, lack of respiratory depression and pacemaker effect), in various surgical operations (including cardiac surgery), as well as in endoscopic procedures, cardiac catheterization, minor surgical procedures, bandages, including in dental, ophthalmological and otolaryngological practice, as well as in obstetric practice during cesarean section.
Ketamine is administered intravenously (jet or drip) and intramuscularly. Adults are administered intravenously 2 .. 3 mg / kg, intramuscularly 4 .. 8 mg / kg. To maintain anesthesia, injections are repeated (0.5 .. 1 mg / kg IV or 3 mg / kg / m). The maintenance of anesthesia by continuous intravenous infusion is achieved by its administration at a rate of 2 mg / kg per hour (on isotonic sodium chloride solution). Ketamine can be used in combination with neuroleptics (droperidol, etc.) and analgesics (fentanyl, promedol, etc.) In these cases, the dose of Ketamine should be reduced.
Ketamine causes an increase in blood pressure (by 20-30%) and increases the heart rate with an increase in the minute output of the heart; peripheral vascular resistance decreases. The stimulation of cardiac activity can be reduced by the use of diazepam (sibazone). As a rule, Ketamine does not cause bronchospasm and laryngospasm, does not inhibit reflexes of the upper respiratory tract. Nausea and vomiting usually do not occur. Ketamine use may be accompanied by involuntary movements, hypertension, and hallucinatory phenomena. These effects are prevented or relieved by the use of tranquilizers or droperidol.
With intravenous Ketamine administration, pain and redness along the vein are sometimes possible; upon awakening, psychomotor agitation and relatively long disorientation are possible.
Clinical data and animal studies suggest that PCP and its analogues have significant narcotic potential and, when used chronically, cause tolerance and physical dependence. All drugs in this class have the property of causing repeated use, which is confirmed by the fact that they are able to support self-consuming behavior in laboratory animals.
The dependence of action on structure
The relationship between the structure and activity of PCP-like substances has been studied in animals. These studies concerned the effect of substitution in the PCR structure on effects such as rotator activity and PCR-like suppression of laboratory animals. Such studies have shown that individual changes in the structure of substances cause changes in their pharmacological activity. Substitution of the piperidine ring with an ethylamine group, leading to PCE, increases the activity of this compound by 6 times. The activity also increases when the benzene ring is replaced by a 2-thienyl group (TCR). The transition from PCR or TSR to TSR or TSR only slightly reduces the activity of substances. The contraction, expansion or cleavage of the cyclohexane ring leads to a sharp reduction in the activity of the substance.
Routes of use and dosage forms
Phencyclidine and its analogues are distributed in various dosage forms, including tablets, capsules, chewing gums, liquids, and powders. Below are photos of packages with PCP seized in Western Europe and the USA.
The most common form of it is plant material such as marijuana, tobacco, parsley or mint leaves soaked in a solution or powder of the drug, which is consumed through smoking. Less common dosage forms of PCP are tablets and capsules containing from 1 to 7 mg of the drug.
Pieces of filter paper, measuring 1 by 1 cm, were seized on the territory of Russia, on which an uncleaned reaction mixture containing PCP was applied in the form of dirty spots.
PCP and its analogues are consumed by mouth or inhalation. The inhalation route of administration is considered preferable, as it allows for more accurate dosing of the drug. This method is carried out when smoking plant material soaked in the substance. As a result, in addition to PCR itself, such products of its thermal decomposition as 1-phenylcyclohexane, piperidine and N-acetylpiperidine enter the human body. Rarely considered drugs are used intravenously.
Ketamine is usually consumed intramuscularly, intravenously, intranasally, and orally. The time of onset of the effect depends on the dose and route of administration. For example, with oral administration, the effect occurs after 15 to 30 minutes, and with intravenous administration, the effect develops at the tip of the needle. In this case, the dose of the drug with the latter method should be 1.5 – 2 times lower. It is believed that with the oral method of administration, a dose of 150-175 mg causes a pronounced psychedelic effect in beginners. The usual doses are 300-350 mg for women and 350-375 mg for men of average weight.
Pharmacokinetics
The pharmacokinetics of these drugs in humans have been studied exclusively on PCP and ketamine. Based on the fact that PCP analogues have a similar structure and have a similar effect on humans, it can be assumed that they have similar pharmacokinetic parameters. Based on ethical standards, such studies were conducted on volunteers who took a dose of PCP by mouth or intravenously, as well as on the basis of data obtained from people admitted to intensive care due to its intoxication.
PCP is rapidly absorbed and widely distributed throughout the body. This applies to the oral (through the mouth), percutaneous (through the skin), pulmonary (through the lungs) and intronasal (through the nose) routes of administration. With an internal intake of 1 mg of PCP by volunteers, its oral bioavailability averages 72% with a range from 50 to 90%. Due to the pyrolytic degradation of PCP into 1-phenylcyclohexane and pyrrolidine, only one third of its dose in a cigarette is available for adsorption. However, it is completely adsorbed in the lungs. Due to its high lipophilicity, PCP has a large volume of distribution, which at an oral dose of 1 mg is approximately 6.2 ± 0.3 l/kg. The blood/plasma ratio for PCR is approximately 1, and the percentage of protein binding is about 65%. The large volume of drug distribution determines its rapid transfer from the blood to various organs, mainly those containing a large amount of fat: the liver or brain. The time of semi-elimination of PCP from the blood is estimated from 7 to 46 hours, and in the case of severe intoxication from 1 to 4 days, which can be explained by the reverse release of it from the accumulated tissues back into the blood.
The main pathway of phencyclidine metabolism in humans is oxidative hydroxylation of the piperidine and cyclohexyl rings under the influence of the cytochrome P450 system. The resulting metabolites (1-(1-phenylcyclohexyl)-4-hydrolxypiperidine and 1-(1-phenyl-4-hydroxycyclohexyl)-piperidine, respectively) make up the bulk of the dose of PCP excreted in urine. It can also contain a dihydroxyl metabolite of the drug: 4-(4`-hydroxypiperidine)-4-phenylhexanol. All of these 3 metabolites are excreted in the urine as conjugates. In addition to those already indicated, 5-N-(1`-phenylcyclohexylamino)-valerian acid can be detected in urine.
The human dose of PCP is excreted in the urine as hydroxylated, conjugated metabolites. With a single intravenous dose of 1 mg, 30 to 50% of the dose is eliminated within 7 days and 77% of the dose is eliminated in 10 days. Only about 2% of this dose is excreted in the faeces. The excretion of PCP in the urine strongly depends on its pH. Acidification of urine promotes the elimination of PCP.

Toxicology
Clinical manifestations
Individuals with acute low to moderate intoxication from PCP obtained by smoking or inhaling 5 to 15 mg of the drug show major behavioral and physiological disorders. They are often symptomatically similar to functional psychosis. Patients often experience visual, auditory, or tactile hallucinations. Psychotic reactions can last for several hours or weeks up to one month. The following symptoms of catatonia may occur: loss of speech, grimacing, immobility, loss of a sense of perspective, in which the world is perceived as two-dimensional. Mental disorientation is often accompanied by alternating periods of lethargy and periods of unreasonable fear. At the same time, patients exhibit behavioral disorders such as completely unnecessary and indecent acts, violence, agitation and anxiety, hallucinations. Observed physiological effects: tachycardia, increased breathing rate, increased blood pressure, excessive tearing, salivation and sweating, redness of the skin, nausea, miosis (pupil constriction), double vision, ataxia, tremor, muscle weakness, urinary incontinence and others.
Individuals who have received 25 mg or more of PCP fall into a comatose state and do not respond to pain. Coma may occur suddenly or as a result of inappropriate behavior of the patient. The duration of coma is usually from 2 to 24 hours, rarely for 5-7 days. Sometimes coma can be caused by respiratory disorders and sleep apnea.
Prolonged PCP abuse is associated with personality changes, not with its side effects. As the drug continues to be used, tolerance to it develops, which makes it necessary to constantly increase its dosage. Over time, people who abuse PCP develop increased irritability, aggressiveness, constant readiness for a fight and antisocial behavior, and depressed mood. Memory, judgment, and cognition are severely impaired by the chronic use of this drug. As a result of abrupt withdrawal from the drug, chronic users experience withdrawal symptoms. The same syndrome can occur in newborns whose mothers have abused drugs during pregnancy.
Clinic of phencyclidine overdose and withdrawal syndrome due to its deprivation.
Phencyclidine is a drug with a multifaceted effect. It is an agonist of CNS receptors for excitatory amino acids, depending on the dose it can have anticholinergic and dopaminergic effects, exhibits alpha-adrenergic and anticholinesterase effects. Therefore, when taking phencyclidine, a variety of clinical symptoms occur, from euphoria and disorientation to catatonia and stupor. It is believed that phencyclidine blocks the reuptake of dopamine, norepinephrine and serotonin, and also interacts with its (phencyclidine part) glutamate receptor, thereby blocking the ligand-controlled channels of this receptor.
The initial signs of intoxication are manifested by euphoria and anger, then ataxia joins, stunning syndrome develops; after which, with an increase in the dose of the drug, catatonic arousal, prolonged oneurism, extremely aggressive behavior caused by “seen” pictures, panic attacks, the development of hypertension and complications of intoxication occur. An important property of the drug is the dulling of pain.
Among the complications of intoxication are:
– neurological (severe headache, visual acuity, diplopia, nausea, vomiting, nystagmus, choreoathetosis, intracranial hypertension, cerebral hemorrhages, tonic-clonic seizures);
– from the cardiovascular system (generalized vasospasm, collapse);
– hyperthermia and rhabdomyolysis;
– acute renal failure.
The main cause of death when using phencyclidine is due to injury, as patients do not feel pain. Pharmacokinetics of phencyclidine. A small dose of the drug is 1-10 mg of phencyclidine, average doses are 5-20 mg, high doses are up to 1 gram. One of the metabolites of phencyclidine is 1-piperidine-cyclohexane-carbonitrile (which can also be found in the initial preparation, since it is a precursor of phencyclidine). When phencyclidine is biotransformed in large doses, prussic acid is formed, which can bring about corresponding changes in the clinical picture of phencyclidine intoxication (in the form of nausea, vomiting, abdominal pain, diarrhea, seizures, coma). The product of the final degradation of phencyclidine is piperidine, which has the smell of fish. There are many structural homologues of phencyclidine that are produced during synthesis (one of them is ketamine).
Some indicators of the pharmacokinetics of phencyclidine: pK = 8.5 (a decrease in the pH of urine to the acidic side significantly increases the clearance of the drug) ; Vd = 6 lkg; T0.5 for more than a day; Ae24 in urine of only 9% unchanged. Lethal concentrations in blood plasma range from 0.3 to 12 micrograms /ml, concentrations causing acute intoxication psychosis average 0.6 micrograms / ml. Phencyclidine easily penetrates the placenta and its half-life in the fetus is longer than T0.5 in the mother’s body.
Treatment. You can not stay “one on one” with the patient, you should not talk to him, so as not to provoke aggression. Preferably, women should work with female patients, and vice versa, men should work with men.
Of the treatment methods: sedation, rehydration (when urine is acidified with ascorbic acid, patients without depression of consciousness, at the rate of 2 grams per the first 400.0 ml of isotonic sodium chloride solution. Repeat the procedure every 6 hours, measuring the pH of urine) and symptomatic treatment.
The patient should be consulted by a psychiatrist and, if necessary, transferred to a psychiatric hospital. Withdrawal syndrome due to phencyclidine deprivation is manifested by aggression, episodes of depersonalization. Tolerance is developed to phencyclidine. Unlike marijuana (see below), productive contact is not possible without pharmacotherapy.
From the Encyclopedia of Psychedelics by P. Stafford.
CONTROVERSIAL COMPOUNDS
Protein-like compounds (delirium). Yohimbine (a stimulant). Kava-kava (intoxication). Ketamine (anesthesia). Nitric oxide (ineffable).
The nine groups of compounds that we have described, which cover more than 100 plant alkaloids and synthetic compounds, are the main psychedelics currently known. But psychoactivity has been discovered or suspected in at least 120 more plants of the New World and 20 plants of the Old World (91 plants are on the list of Schultes and Hoffmann), and the substances isolated from them encourage chemists to synthesize and test new compounds. Efron outlined this work of chemists (Sandoz, Ciba, Hofmann-LaRoche, and the National Institute of Mental Health participated in the work). The first edition (1968) listed 590 substances and references to literature on them. In 1972, Efron expanded the list to 1,555 compounds (including barbiturates and tranquilizers).
BELLADONNA-LIKE COMPOUNDS
The family Solenaceae, which includes more than 2,400 species, is worth special attention. Many of them contain alkaloids such as atropine and scopolamine. Atropine is found in mandrake root, henbane and stinky datura; it is about 4.5% in the Asthmador asthma drug. Schultes and Hoffmann state that there is not a single report of the effect of atropine, “which could explain the presence of belladonna in the witchcraft potions of medieval Europe.” However, Hoffer and Osmond cite several historical cases that attest to her psychoactivity. One such case occurred in a family of five people who ate tomatoes vaccinated for stinky dope. On average, one tomato contained 6.36 grams of atropine. “All five developed delirium of varying depths, and some of them spent several days in the hospital. Apparently, this is the first known example of hallucinogenic tomatoes.”
The effect of the substance is indicated by both names – atropine and belladonna. The first comes from the word “Atropos” – one of the three goddesses of fate in Greek mythology – due to the fact that in the Middle Ages belladonna was used as a poison. The second indicates the ability of atropine to dilate the pupils of “beautiful ladies.” Both are currently used in medicine as anticonvulsants, especially for Parkinsonism; the average dose of atropine is 0.5 mg. Users can withstand doses of more than 1 g, but in most cases it has a toxic effect if the dose exceeds 10 mg.
Probably the most important chemical compound in belladonna is scopolamine. It is found not only in the plants already mentioned, but also in the root of several trees of the genus Datura, which are consumed by the aborigines. Scopolamine was used in both hemispheres: in the Middle East it was combined with cannabinols, in the Andes it was added to the San Pedro cactus with a mescaline-like effect. Scopolamine accounts for 50.4% of Asthmador. Tim Leary says he’s never heard of a good belladonna trip; my own experience is an exception (so far).
YOHIMBINE
This “psychedelic stimulant” is obtained mainly from the bark of the West African tree Corinanthe yohimbe or Pausinystalia, although it is also found in other Corinanthe species, Aspidosperma quebranchoblanco and Mitragina stipulosa. If you drink this alkaloid while brewing tea, the effect begins after 45-60 minutes. It is reported that the effect occurs faster in combination with 500 mg of vitamin C.
Yohimbine dilates blood vessels and improves peripheral blood supply along with stimulation of the spinal ganglia, which control erection. This is accompanied by a mild “hallucinogenic effect” that lasts about 2 hours. Then the user can fall asleep completely peacefully.
An article in the High Times highlights that yohimbine is a MAO inhibitor that alters the metabolism of adrenaline and other substances, so it should be used with caution.:
“people with kidney and heart problems or diabetics should not experiment with yohimbine. Moreover, yohimbine should not be used together with mescaline, LSD, MDA, MMDA or amphetamine… Do not consume chocolate, cheese, sherry, bananas, pineapples, sauerkraut, or other foods containing tryptophan for 12 hours before and after taking this medicine. This combination can lead to a dangerous increase in blood pressure and shortness of breath.
In the worst case scenario, seek medical help. Yohimbine is legal, so such a visit will not cause any complications. The best antidote for yohimbine poisoning is sodium amytal, but let the doctor do it. Self-medication during a panic is very dangerous. Most people overdid it.”
To obtain yohimbine, you need to boil the bark to leach out the psychoactive components, after which the original product can be discarded. The effect on the psyche is very mild. Adam Gottlieb comments on the ability of yohimbine to act on the sexual sphere, for example, on erection in males:
“Another action that gives pleasure is a warm flutter in the back, which is especially pleasant during copulation and orgasm (it seems that the bodies merge with each other), mental stimulation and increased emotionality and sexuality …”.
KAVA-KAVA
Kava-kava is the pulp of the roots and the lower part of the trunk of a tall perennial shrub that grows naturally on the islands of the South Pacific Ocean. It is mentioned by a Swedish botanist who accompanied Captain Cook on his first voyage to the Hawaiian Islands (1768-1771). In 1886, Louis Lewin carefully studied this plant.
Kava kava (Piper methisticum) grows well at sea level in the Solomon Islands, Fiji, Samoa, Tahiti and New Guinea. If there is enough sun, the plant can reach 20 feet. Psychoactive compounds are found in the root, which after 3-4 years reaches 3-5 inches in thickness. The roots of older plants become heavy and gnarled, accumulating strength and fragrance. After 6 years, such a root can weigh 20 pounds, and after 20, as much as 100!
When harvested, these roots are peeled, cut into pieces, which are then chewed (using the tongo method) or ground between stones (using the Fijian method). Norman Taylor perfectly describes the preparations that result in a grayish-brown or whitish liquid; most users consider it to be similar to soap, spicy and numbing. Hoffer and Osmond write that in Hawaii, “the nobility uses it for pleasure, priests use it in ceremonies, and the working class uses it to relax. It is given to mediums and soothsayers to increase their psychic power. It is used to inspire and promote contemplation. It seems to be used in the same way that some users use LSD and psilocybin.”
Interestingly, many people in the South Pacific stop drinking alcohol after becoming familiar with kava kava. Everyone agrees that it does not leave a hangover and causes a carefree and happy state without any physical or mental stimulation. According to Lewin, “this is a real euphoric, which first makes speech smoother and more lively and sharpens the hearing. The subjects were never irritable, aggressive, or violent.” If kava-kava is taken regularly and in large quantities, “the legs become tired and weak, the muscles are poorly controlled, the gait becomes unstable, and in general such a person gives the impression of being drunk.” But mental changes usually bring pleasure with an abundance of absolutely magical sensations. A surprising number of visitors to Hawaii believe that kava kava is superior to champagne.
If you take a large amount, the visions are destroyed, the pupils dilate and it is difficult to stand on your feet. If kava-kava is consumed frequently and in large quantities, the skin begins to peel off.
Kava-kava is not prohibited. You can buy it in plant stores and receive it by mail. The fresher it is, the more powerful it is.
The effect of kava-kava on the psyche is caused by at least six a-pyrons: kavain, dihydrokavain, metisticin, dihydromethysticin, yangonin and dihydroyangonin. None of these resins are soluble in water. Therefore, Gottlieb says, you need to prepare an aqueous emulsion or an emulsion in coconut milk.:
“After chewing the root, as they do on the islands, and adding a little oil of Provence and lecithin, mix in a mixer. To get this mixture, place 1 ounce of powdered kava kava, 10 ounces of water, 2 tablespoons of coconut oil or olive oil, and 1 tablespoon of lecithin granules (from health food stores) in a stirrer and mix until a milky liquid is obtained. This amount is enough for 2-4 people.”
KETAMINE
Ketamine, described by Hitchcock, is a non-barbiturate anesthetic that is notable for its lack of side effects. It is also called ketalar (ketamine hydrochloride). It is mainly used for pediatric anesthesia. Intramuscular injection of 9-13 mg / kg of body weight causes anesthesia after 3-4 minutes, it lasts 12-25 minutes. An intravenous infusion of 2 mg/ kg usually causes anaesthesia after 30 seconds, which lasts 5-10 minutes.
When it was given to adults for experimental purposes, some patients stated that their experiences were similar to psychedelic ones. Experiments soon showed that if the usual dose is reduced by 10 times, ketamine causes a trip lasting 45-60 minutes. Many subjects noted that if they lie quietly under the influence of this substance, it can lead to spiritual experiences.
Pack-Davis notes that approximately 12% of the more than 10,000 patients who were given this drug in 105 separate studies had unpleasant “critical reactions.”:
“PSYCHOLOGICAL MANIFESTATIONS VARY IN STRENGTH BETWEEN A PLEASANT DREAM-LIKE STATE, VIVID IMAGES, HALLUCINATIONS AND SEVERE DELIRIUM, IN SOME CASES THESE STATES ARE ACCOMPANIED BY CONFUSION, AGITATION AND INAPPROPRIATE BEHAVIOR, WHICH SOME PATIENTS CONSIDER AN UNPLEASANT EXPERIENCE, THE ACTION USUALLY LASTS NO MORE THAN A FEW HOURS, BUT IN SOME CASES IT MANIFESTS ITSELF 24 HOURS AFTER SURGERY. NO RESIDUAL EFFECTS OF KETALAR ON THE PSYCHE WERE FOUND…
THESE (CRITICAL) REACTIONS CAN BE REDUCED IF VERBAL, TACTILE, AND VISUAL STIMULATION IS MINIMIZED DURING THE RECOVERY PERIOD…”
People who have experienced low doses report feelings unrelated to normal, ordinary reality. Blood pressure and pulse often rise, hypotension and bradycardia are observed. “Ketamine has wide safety limits,” says the Pack-Davis brochure, “in several cases, unintentional ingestion of too high a dose of ketalar (up to ten times) was followed by a prolonged but complete recovery.”
The reaction to low doses is illustrated by this story:
“the experience of music was on the rise. Because I am a musician, my friend, knowing this, chose music. Everything was fine with both eyes open and closed, it didn’t matter yet. Closing my eyes was better. I started feeling disoriented, and when I closed my eyes, I started getting a lot of information. Colors, shapes, interweaving of feelings. Sounds and inner visions got mixed up.
I sank deeper and deeper into this state until the world disappeared. I was no longer in my body. I didn’t have a body.
I had reached a certain point where I knew I was going to die. There were no questions, no “what if I want to” or “probably I will want to”, what an incredible feeling it was!
Then I reached a point where I was ready to die. There was no question of choice, there was only a wave that lifted me higher and higher, and at the same time there was what I would normally call the fear of death. I began to realize that, after all, there was nothing special about the fact that I had foreseen death. Except that I knew it was death, that something was dying.
I’ve reached this point where I’ve just dropped it all. I just screamed, and then I entered a space where there were no words. I’ve said the same word thousands of times, starting with Buddha. I mean, “oneness-with-the-universe,” “cognition-of-one-own-divinity”-all these words I used later to explain my experiences.
I felt like I was “at home.” I didn’t want to go anywhere, and I didn’t have to. I was in a blissful state that I had never experienced before.
I stayed here for a while, and then I came back. I didn’t want to go back. The deep state lasted no more than half an hour.
When I told my guide about this, highlighting a few words about the experience, he said: “Yes, what happened and happens to others, and what you finally got rid of, is the feeling of deep disappointment that we carried out from childhood. Now it’s been crossed out somehow.” It was a feeling: I was relieved of a deep disappointment. My heart was no longer aching. It was like, “Phew!!!” The effect turned out to be long-lasting-it really lasted and was sustained by similar experiences-not necessarily on any drug after all.”
In 1978, Moore and Altaunien published a book about ketamine. John Lilly also spoke well of him, calling this compound “K.” Some people were enthusiastic, and some were disappointed. Here is the story of one of the subjects, who decided that his experiences were completely different from those caused by other psychedelics.:
“Speaking of my ketalar ordeal, I do not know-and I have no doubt that someone might know-how to describe it. It has the same connotation as Lilly’s experience described in his autobiographical book, that is, contact with Someone who does earthly things. (Before my ketalar test, I had not read Lilly’s book and entered the experience without any definite expectations. If I expected anything, it was something similar to the effect of other psychedelic substances. I was less interested in God-related experiences than a complete loss of awareness. Even in the deepest experiences with LSD, I still had something similar to the ego, but here there was such a complete annihilation of consciousness that what connected me to It in a mystical experience simply disappeared).
A pleasant surprise: I found that I can remember the details of my experiences better than in the experiments with LSD and mushrooms. It was like going to the movies. It was more than just a conceptual story. The plot is easier to remember than comprehension. On LSD and mushrooms, I could always talk and felt that if I wanted to, I could even write, but with ketamine, there were no eyes, no hands to write, there was no world in which to perform such an action.
Ketamine is the only drug that has exhausted me. I passed out 10 minutes after its effects ended, and immediately swore that I would never take it again. Of course I won’t.
My experiences have shown that this is the most hallucinogenic drug I have ever taken. It’s like I’ve been given something more personal and significantly less meaningful than the Basis of Being.”
The ketamine molecule is very similar to the molecule of PSP, an analgesic anesthetic, which is used in veterinary medicine. The chemical name of PSP (PCP) is phencyclidine or benactazine. Pak-Davis supplies it under the name “Sernil”. It was presented as THC called “pig”, “greens” and “Pills of Peace”. In smoking mixtures, it is often called HELL (“Angel Dust” – “angel dust”). Taking PSP too often causes a “shift” or “schizo” and is not recommended. Domino, Lubi and Kovacic made the best report in the scientific literature. The entire issue of the Journal of Psychedelic Drugs for January-June 1978 was devoted to PCR (12 articles).
NITRIC OXIDE
It was first synthesized in 1772 by Sir J.Priestley. In 1799 Davey found that nitric oxide (N2O) relieves pain. Since the second half of the 19th century, it has been used as an anesthetic for tooth extraction, childbirth and some operations. To date, it is not clear how this molecule of just three atoms causes anesthesia, fun, and other deeper effects. Many users have no doubt that nitric oxide facilitates access to the “unconscious.” It also seems to some that it leads to the state of recognition described by Andre Breton.:
“This is a special state of the brain in which life and death, reality and fantasy, past and future, expressible and inexpressible, high and low cease to feel like contradictions.”
The dose is easy to adjust. After cessation of inhalation, the effect stops, and the gas is completely eliminated after 5-10 minutes. Unlike the previously described psychedelics, the effects are not amenable to further description. The user retains an impression of the experience rather than an accurate memory.
In no case should you forget about oxygen, otherwise you may suffocate. It’s probably a good idea to mix nitric oxide with air.