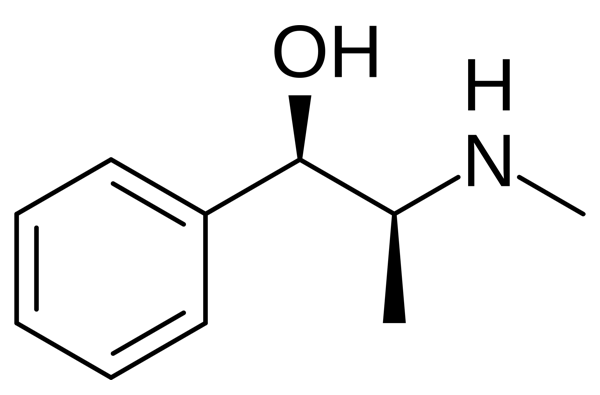
Ephedrine
1-Phenyl-2-(methylamino)Propanol-1 can also be attributed to amphetamine derivatives.
Ephedrine is an alkaloid found in various ephedra species of the ephedra family, including Ephedraequisetina (horsetail ephedra), which grows in mountainous regions of Central Asia and Western Siberia, and Ephedramonosperma, which grows in Transbaikalia.
Ephedrine is white needle-like crystals or white crystalline powder with a bitter taste. Easily soluble in water, soluble in alcohol. It was first isolated in 1887. The chemical structure of ephedrine differs from adrenaline in that it does not contain hydroxyls in the aromatic cycle; instead of an aminoethanol chain, ephedrine contains an aminopropanol chain.
Until recently, ephedrine was obtained exclusively from natural raw materials – various types of ephedra. However, insufficient amounts of wild-growing ephedra, whose reserves are constantly decreasing, have necessitated the development of methods for producing synthetic ephedrine suitable for industrial use.
One of the methods of synthesis of ephedrine, the synthesis of Furneaux and Kanas, comes from benzaldehyde, which is condensed with nitroethane to form 2-methyl-2-niro-1-phenylethanol. It is reduced to an amine, which is a mixture of geometric isomers that can be separated by fractional crystallization. The desired isomer is methylated, resulting in ephedrine. As for the other isomer, it turns into ephedrine when boiled with dilute hydrochloric acid.
The biochemical synthesis of ephedrine is also described, which consists in the fermentation of glucose by yeast carbolygase in the presence of benzaldehyde, which is converted into 1-phenyl-2-ketopropanol. And when it is reduced in a methylamine solution, it gives ephedrine.
Catecholamines
Catecholamines are physiologically active substances that act as chemical intermediaries and “control” molecules in intercellular interactions in animals and humans, including in their brains.
Catecholamines: adrenaline, norepinephrine, and dopamine are synthesized in the adrenal medulla, in the sympathetic nervous system, and in the brain. Since catecholamines and their metabolites, such as methanephrine and normetanephrine, are secreted in increased amounts in various diseases, they can be used for diagnostic purposes. In a number of mental illnesses, there is a lack of catecholamines in certain areas of the brain.
Adrenaline
Synthetic adrenaline is used as a drug under the name “Epinephrine”.
The artificial synthesis of adrenaline was carried out for the first time by Friedrich Stolz and, independently of him, H.D. Dakin. In 1904, the laboratory of the pharmaceutical company Holkest established the industrial production of adrenaline by chemical synthesis, which began to be produced under the trade name Supra-renin. The advantage of the artificial drug was that, due to the consistency of its composition and purity, it could be accurately dosed. In addition, it was better stored and did not have many of the side effects of natural adrenaline preparations. The synthesis of adrenaline soon led to the establishment by Ernst Joseph Friedmann in 1906 of its exact structural formula.
The synthesis of adrenaline is carried out by the interaction of pyrocatechin with monochloroacetic acid chlorohydride. The product formed in the first stage reacts with methylamine to form adrenalon, which is reduced and a mixture of optical isomers of adrenaline is obtained. The racemate is divided into antipodes using tartaric acid:
Epinephrine and norepinephrine are derivatives of a fairly strong reducing agent, pyrocatechin, and therefore are easily oxidized by sodium nitrite, iodine, potassium iodate, and other oxidizing agents. This is used in pharmacopoeial analysis to determine authenticity: the red oxidation product of epinephrine is formed at pH 3.6, and norepinephrine is formed only at pH 6.5. These color reactions can also be used for the quantitative determination of drugs by photometric method.
Adrenaline is used to increase blood pressure, stimulating the vasoconstrictor mechanism of the circulatory system and accelerating the activity of the heart. It is used topically to stop bleeding and to enhance the effect of local anesthetics, subcutaneously to relax bronchial muscles in asthma and anaphylaxis, intravenously to overcome acute circulatory collapse.
Ephedrine
1-(benzo-(1,3)dioxol-5-yl)-N-methylpropane-2-amine
Another prominent representative of a number of phenylethylamines is 1-(benzo-(1,3)dioxol-5-yl)-N-methylpropan-2-amine. It is also called MDMA (3,4-methylenedioxy-N-methamphetamine). MDMA has a unique psychoactive characteristic due to its ability to induce feelings of euphoria, intimacy and trust towards other people, as well as reduce feelings of fear and anxiety. These emotional effects have a very stable manifestation, noticeably distinguishing and distinguishing MDMA from other stimulants and psychedelics in a separate group of empathogens.
MDMA was first synthesized in 1912 by German chemist Anton Kelisch, who worked for the pharmaceutical company Merck. Patent number 274,350 describing the synthesis of this substance was issued on May 16, 1914 in the city of Darmstadt, Germany, but was soon forgotten. Researchers at that time did not know or assume any special properties of the new chemical compound, and this patent described it only as a byproduct in the synthesis of hydrastinin.
Only about half a century later, the MDMA molecule again attracts the attention of researchers, already as a psychoactive compound. Secret research in the 1950s in the US Army included experiments with MDMA, where this substance was codenamed EA-1475. The purpose of these studies was to find new ways to manipulate consciousness. However, the army has not tested MDMA directly on humans, limiting itself to animal experiments only.
The effect on humans was established only in the late 1970s thanks to the work of Alexander Shulgin, a well-known researcher of psychoactive substances. In 1967, on the advice of one of his students, Shulgin synthesized and tested MDMA on himself, using a careful method with a gradual increase in dose. In the first scientific articles on MDMA, published in 1978, Dr. Shulgin, in collaboration with colleagues, described the effect of MDMA on the human psyche as “an easily controlled altered state of consciousness with emotional and sensual shades.” Soon after, MDMA became the most popular medication among psychotherapists. Very soon, the use of MDMA spread beyond clinical practice. In 1985, MDMA was outlawed, and since then MDMA has been criminalized by the laws of most countries and international conventions on the restriction of trafficking in psychoactive substances. The production, storage, transportation, and distribution of MDMA is a criminal offense in most jurisdictions around the world. In addition to recreational use, before its prohibition, MDMA was actively used as an adjunct in psychotherapy, especially for counseling couples and resolving family problems and conflicts.
In recent years, MDMA has been returning to official medicine. Research organizations in the United States, Switzerland, Israel, and the United Kingdom are developing methods for using MDMA as a psychotherapeutic drug for the treatment of post-traumatic mental disorders.
Even oncologists became interested in 1-(benzo-(1,3)dioxol-5-yl)-N-methylpropan-2-amine. In 2006, scientists from the University of Birmingham demonstrated that MDMA is able to stop the growth of cancer cells. However, to achieve this effect, the patient must take a lethal dose of the drug. In a study published in 2011, scientists from the University of Western Australia reported that they had made some changes to the molecule and the effectiveness of the resulting drug had increased 100 times. MDMA research is currently ongoing.
MDMA is synthesized mainly from safrol. Safrol (3,4-methylenedioxyphenylprop-2-ene) is a colorless or yellowish oil obtained from the bark of the sassafras shrub root.
Thus, 1-phenyl-2-aminopropane is the ancestor of a whole group of amphetamines. That is, there are a large number of derivatives of this molecule, most of them have a psychostimulating effect to a greater or lesser extent. Although substances of this type are prohibited in most countries, it is quite difficult to conduct research in this area, but still the study of amphetamine derivatives is very relevant. Since they have serious biological activity and are analogues of substances contained in the environment, in the body.
For example, in recent studies, when studying the sleep and awakening of winter-sleeping animals, it turned out that “when studying the awakening effect of phenylalkylamines (phenamine, pervitin, ephedrine, sympathol, and adrenaline) and analeptics (corazole, cordiamine, and strychnine) on intact and sympathectomized winter-sleeping animals, we have established the following: sympathomimetic amines (phenylalkylamines) and analeptics are capable of interrupt even this type of sleep, which is the torpor of winter-sleeping animals. It turns out that work in this field is not without meaning and may contain a great many more necessary, or even necessary, properties.