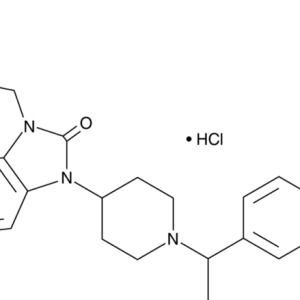
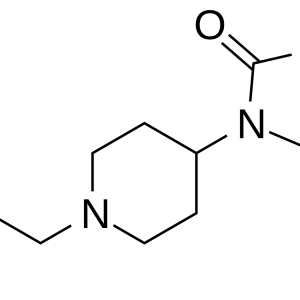
Given the centuries-long history of the use of opium and its alkaloids, it was only in 1973 that the presence of membrane receptors called “opioid receptors” in the CNS was experimentally proven. A little later, the first substance that stimulates endogenous opioid receptors was discovered in the organism and named enkephalin (from the word “kephale” – head). Currently, 3 families of endogenous opioids are distinguished:
-endorphins,
-enkephalins,
-dynorphins.
All endogenous opioid peptides are formed from protein precursors produced in different ratios in the nervous system and peripheral organs. Along with pain suppression, endogenous opioids participate in the regulation of GI functions, internal secretion glands, heart, higher nervous activity, etc. The biological effects of endogenous opioids are realized through stimulation of opioid receptors localized both in the CNS and in peripheral tissues. At least five types of receptors are currently known:
μ (mu) – receptors,
κ (kappa) receptors,
δ (delta) – receptors,
σ (sigma) – receptors,
ε (epsilon) – receptors.
Each of these types has a specific location and physiological significance . Meanwhile, the main physiological task of opioid receptors is to maintain adequate antinociception by suppressing the functions of the nociceptive system.
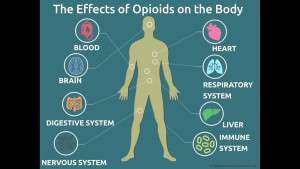
Opioid receptors
In cases where the antinociceptive system fails or is unable to fully correct the work of the nociceptive apparatus (for example, with severe acute pain or chronic pain syndromes), the latter can cause serious damage to human health and quality of life. At the same time, the elimination of pain or its relief becomes the main task of the doctor. Currently, substances derived directly from opium (for example, morphine) are called opiates, and substances with similar properties, including synthetic and semi-synthetic origin, are grouped into opioids. All these substances make up a large group with the traditional name narcotic, or opioid analgesics. It is more correct to call these substances opioid analgesics. The term narcotic comes from the Greek. “narcoo” – to numb, put to sleep, cause reversible loss of consciousness. Opioid analgesics in therapeutic doses cause pain relief without falling asleep and loss of consciousness. Therefore, the terms “narcotic” and “narcotic analgesic” are not quite appropriate. Currently, there is an intensive search for new opioid analgesics that are not inferior in strength to the analgesic effect of morphine, while devoid of its properties to cause respiratory depression, euphoria and addiction. It was in the process of these searches that the antagonist agonists appeared. For example, stimulation of the µ – opiate receptors of the respiratory center leads to asphyxia, therefore, blockers of these receptors are safe in this regard. In addition, the psychomimetic effect (euphoria) of many opioids is also associated with the stimulation of m – receptors, which is the basis for the development of mental and physical dependence. At the same time, m –receptor blockers, on the contrary, often cause dysphoria, which means that their narcogenicity is less pronounced. By modifying the molecules of opioid analgesics, not only the pharmacokinetic properties of drugs change, but also the affinity for certain types of opiate receptors, as well as the nature of the effects of opioids on them. In this regard, opioid analgesics are divided into three groups according to the type of effect on receptors:
-Morphine–like substances (pure agonists) are opioid analgesics that stimulate all types of opiate receptors
-Antagonist agonists, or partial agonists– are opioid analgesics that stimulate one type of opioid receptor and block another. For example, pentazocine is both a k– agonist and a blocker of the m- opiate receptors.
-Opioid antagonists (complete antagonists) are drugs that block all types of opiate receptors. As a rule, antagonists are used for symptoms of intoxication with opioid analgesics.
Despite the above in favor of agonist – antagonists, morphine still occupies a special position and to this day it justifies the definition given to it by V. Osler: “Morphine is a god in medicine.” Morphine is a universal stimulant of all opioid receptors. There is no more “ubiquitous” analgesic than morphine, which changes all aspects of pain, that is, it not only reduces the intensity of pain, but also eliminates the accompanying phenomena – emotional coloring, motor reflexes and vegetative reactions.
Analgesic effect. Morphine-induced analgesia is caused by stimulation of all types of opiate receptors and is not accompanied by loss of consciousness. The analgesic effect of morphine and other opioids is based on direct inhibition of afferent (ascending) nociceptive pathways coming from the posterior horns of the spinal cord and activation of efferent (descending) antinociceptive pathways going from the midbrain through the medulla oblongata to the posterior horns of the spinal cord. Opioid receptors have been found along these pathways (namely, in the near-conductive gray matter, the reticular formation, the median and large nuclei of the brain stem suture, as well as the spinal cord). Morphine stimulation of these receptors leads to an increase in the output of potassium ions from neurons, a decrease in the input of calcium ions, which causes the development of cell hyperpolarization and inhibition of postsynaptic neurons. In addition, morphine inhibits the release of pain neurotransmitters. Reinforcing action and mood. An equally important feature of morphine is the ability to significantly change the subjective assessment of pain. Against the background of the action of morphine and its analogues, pain tolerance increases, it becomes less uncomfortable. The modulation of pain perception and euphoria observed against the background of morphine administration into the body seems to be carried out within the limbic system of the brain (anterior temporal, frontal cortex, some parts of the hypothalamus), which plays an important role in the formation of emotions. It is in the limbic system that the highest density of opiate receptors, such as μ -, κ – and σ – receptors, has been revealed. These receptors in the limbic system regulate the functioning of the dopaminergic system. The so-called reinforcing effect of morphine and other opioid analgesics is associated with increased release of the dopamine mediator in the limbic system, in the cerebral cortex and hypothalamus. It has been experimentally shown that the appearance of positive emotions, serenity, feelings of peace, languor, and euphoria is associated with stimulation of the μ – and σ – receptors, and the use of drugs with a tropicity to the κ-receptors (e.g., pentazocine, naloxone) causes the opposite effects – dysphoria (apathy, drowsiness, mental experiences, longing grief and fear), since the k – receptors inhibit the release of dopamine. Other central effects. Opiate receptors, which are targets for the effects of opioid analgesics, are found in almost all parts of the nervous system, therefore, it is not surprising that the range of their central effects is very wide. Opioids, like morphine itself, affect the thermoregulation center of the hypothalamus. They tone the heat transfer center and lower the setting temperature, thereby (especially in high toxic doses) causing hypothermia. Morphine reduces the release of hypothalamic releasing hormones, in particular gonadoliberins (stimulating the release of FSH and LH by the adenohypophysis) and corticoliberin (stimulating the synthesis and secretion of ACTH by the adenohypophysis). As a result, the plasma concentration of adrenal cortex hormones and sex hormones is indirectly reduced.
Morphine and other m–receptor stimulants increase prolactin secretion. This happens, apparently, due to the elimination of the inhibitory effect of dopamine (PIF). Under the influence of morphine-like analgesics, the release of the posterior pituitary hormone vasopressin (ADH) increases, as a result of which the volume of diuresis decreases. Morphine and other opioids cause pupil constriction by stimulating the μ- and κ– receptors in the nuclei of the oculomotor nerve in the midbrain. It is important to note that “pinpoint pupils” are an important symptom of opioid overdose. Against the background of high doses of morphine and its analogues, epileptic seizures often occur, especially in children. Stimulators of the μ – and σ – receptors excite certain groups of hippocampal neurons, probably due to inhibition of the release of the inhibitory mediator GABA. Epileptic seizures caused by opioids are stopped by their antagonists, in particular naloxone and naltrexone. Respiratory depression is one of the features of opioids, which significantly complicates their use in the clinic. Thus, morphine and other µ-agonists inhibit breathing at least partially, even in small therapeutic doses that do not affect consciousness. Death from an overdose of morphine and its analogues is caused precisely by respiratory arrest. A large number of opiate receptors – μ -,- κ – and σ – have been found in the parts of the medulla oblongata that regulate vital functions. By stimulating these receptors, morphine depresses the respiratory center, weakens its reaction from the overlying parts of the brain, its sensitivity to carbon dioxide and acidosis. Fentanyl, Alfentanil, Remifentanil, in addition to direct suppression of the respiratory center, cause rigidity of the respiratory muscles – “wooden chest” syndrome. Mixed-action analgesics (agonists-antagonists) disrupt breathing more weakly. The suppression of the cough reflex is due to a decrease in the tone of the cough center, which is also located in the medulla oblongata. Antitussive effect is characteristic of many opioids, in particular morphine, methylmorphine (codeine), ethylmorphine. The latter have proven themselves mainly as antitussive agents. Morphine is used only in extreme cases, when a debilitating cough can lead to death (e.g., tuberculosis or injury). Nausea and vomiting occur with the use of most opioids. Thus, 40% of patients experience nausea when taking morphine preparations, and in another 15% of cases – vomiting. This is due to direct stimulation of the chemoreceptors of the trigger zone of the emetic center located in the medulla oblongata. The effect on the cardiovascular system. Morphine and similar drugs (stimulating m – receptors) cause bradycardia, that is, a decrease in heart rate. Morphine stimulates the vagus nerve nuclei in the medulla oblongata, which leads to increased parasympathetic effects on the myocardium. As a result, the heart rate and strength decrease. In addition, there is reliable data on the presence of opiate receptors in the myocardium itself, which means that substances with a tropicity to them can affect the heart muscle not only by acting on the vagus nerve, but also directly. At the same time, analgesics that stimulate k– receptors, such as pentazocine, buprenorphine, on the contrary, cause tachycardia, which is probably due to their ability to induce the release of norepinephrine by stimulating k– receptors at the ends of sympathetic nerves. Tramadol also causes tachycardia. Morphine causes orthostatic hypotension up to collapse. A decrease in blood pressure occurs due to the fact that analgesics like morphine (especially natural opiates) stimulate the release of the histamine mediator from mast cells. By stimulating histamine H1 receptors in peripheral arterioles and venules, histamine causes their significant expansion. As a result, the OPSS decreases. Lowering blood pressure by opioid analgesics is also potentiated by the development of bradycardia and depression of the vasomotor center in the medulla oblongata. The effect on the gastrointestinal tract. Morphine and other m–receptor stimulants have little effect on gastric secretion. However, in some cases, the secretion of hydrochloric acid may decrease, which is associated with the ability of morphine to suppress the release of acetylcholine from the vagus nerve endings. The effect of morphine-like analgesics on the evacuation of stomach contents is significant. Even small doses of drugs prolong the release of the stomach from the contents up to 12 hours (unlike the norm – 3-4 hours). Several millennia ago, before the discovery of morphine itself and the production of its analogues, even before the detection of analgesic and psychotropic properties in opium, it was the main remedy in the treatment of dysentery and chronic diarrhea. Intestinal tone increases strongly against the background of opioids, which leads to a decrease in propulsive contractions. In addition, opioid analgesics cause an increase in the tone of the sphincters that “separate” various parts of the gastrointestinal tract, including the Oddi sphincter (regulating the flow of bile into the duodenum). As a result of the spastic effect of opioids on the gastrointestinal tract, the passage of its contents slows down and the absorption of water increases, the density of intestinal contents increases. The combination of these processes is characterized by the term obstipation (constipation). The mechanism of obstipation is due to stimulation by opioid analgesics of the µ receptors of the smooth muscle walls of the gastrointestinal tract, as well as a decrease in the release of acetylcholine, prostaglandin E. Morphine significantly suppresses the secretion of digestive juices: the pancreas, the intestinal glands themselves and the gallbladder. Effect on other smooth muscle organs. Morphine causes an increase in bronchial tone, which complicates its use against the background of bronchial obstructive diseases, in particular, bronchial asthma. This property of morphine is due to an increase in the tone of the vagus nerve center, direct stimulation of opiate receptors of the bronchial tree, and, importantly, the release of histamine. As is known, histamine is a powerful bronchoconstrictor, and the mast cells that secrete it have a high density in the mucosa of the upper respiratory tract. Moreover, histamine, locally dilating the vessels, leads to edema, which makes it difficult for air to pass to the alveoli. In therapeutic doses, morphine increases the tone of the ureters, increases the tone of the sphincter and the volume of the bladder. As a result, this leads to a decrease in diuresis and anuria. Sometimes urinary retention is so long that you have to resort to catheterization. The decrease in urinary function of the kidneys is due to direct stimulation by opioids of the μ -, κ – and σ -receptors of the smooth muscle walls of the ureters and the sphincter. In addition, increased release of ADH by the posterior pituitary gland against the background of therapeutic doses of morphine contributes to a decrease in diuresis. Morphine and its analogues reduce uterine tone, so it is not recommended to use them in obstetrics. In addition, they, being highly polypophilic substances, easily penetrate the placental barrier and inhibit fetal respiration. In this regard, newborns are often born with severe asphyxia. However, pentazocine and promedol can be used to anesthetize childbirth. They do not penetrate the placenta, and promedol, moreover, stimulates uterine contractions and dilates the cervix. Other effects of opioid analgesics. Some drugs, especially morphine and opiates, have the characteristic property of causing urticaria and itching of the skin. This is due to the displacement of histamine – an allergy mediator – from mast cells. Histamine causes dilation of skin vessels, redness, swelling, burning and itching. Redness of the face, neck and upper chest often occurs (Freye E., Levy J.V., 2008) (Casy A.F., Parfitt R.T., 1986). The main indication for prescribing opioid analgesics is pain. Unlike all currently known analgesics, they have the most powerful analgesic effect. However, despite this, the factor limiting their widespread use is narcogenicity. Some time ago, opioids were prescribed only for the relief of acute pain, since during the period of its disappearance, the patient did not have time to develop addiction, and the only example of treatment for chronic pain syndromes was chronic oncological pain. Over the past three decades, this statement has been revised, and opioid analgesics have been used to treat chronic pain of non-oncological origin. Moreover, today there are more and more opioid analgesics with low narcogenicity. One of the important factors preventing the development of addiction is the correct selection of patients and medications, a detailed dosage and administration scheme. Typical examples of such pain syndromes are dorsalgia (back pain syndrome caused by dystrophic and functional changes in the tissues of the musculoskeletal system), osteoarthritis and other joint diseases, as well as adhesive disease, Crohn’s disease, chronic pancreatitis, etc. These diseases are not life–threatening, but their leading clinical symptom – chronic pain – leads to disability of patients. Almost all opioid analgesics, when administered repeatedly (for medical or non-medical purposes), can cause the development of tolerance and dependence. Tolerance (addiction) to opioids develops quite quickly (usually after 1-2 weeks of systematic administration) and is manifested by a weakening of the reaction to the drug. This is the reason for the gradual increase in its dose in order to achieve the necessary activity. Addiction is the main reason for drug addicts to use opioids in doses ten times higher than the average therapeutic ones. Therefore, it is not surprising that the most common cause of death for drug addicts is an overdose. Mental dependence usually precedes physical dependence. Mental dependence is the constant or periodic administration of a drug in order to achieve a state characterized by emotional uplift, a sense of pleasure and loss of discomfort.
The main difference between mental dependence and physical dependence is that with the abrupt withdrawal of an opioid, there is no pronounced withdrawal syndrome. Almost all opioid analgesics, when repeated, cause physical dependence, which is characterized by certain behavioral reactions, including the forced need to constantly take a certain drug or substance in order to avoid the development of a serious condition caused by discontinuation of its administration (withdrawal syndrome). The mechanisms underlying the development of physical dependence on opioids remain unclear. It is believed that when systematically administered, opioids interfere with the metabolism of brain mediators. The abrupt withdrawal of the analgesic “ricochet” shoots a compensatory increase in the synthesis of neurotransmitters, which is manifested by withdrawal syndrome. The first messengers of withdrawal syndrome are lacrimation, runny nose, yawning, chills, anxiety, followed by diarrhea, shortness of breath, nausea and vomiting, severe pain in muscles, lower back and joints, spastic muscle contraction. A very specific triad of signs of opioid withdrawal is mydriasis (pupil dilation), “goose bumps” and dehydration, due to the loss of a large volume of fluid during vomiting, diarrhea and sweating. Withdrawal syndrome can be caused not only by the withdrawal of opioid analgesics, but also by the introduction of opioid antagonists. This is due to a decrease in the effect of the analgesic on the receptors, through their blockade with naloxone / naltrexone. In narcology, the method of “precipitated withdrawal syndrome” is used, based on the identification of hidden opioid dependence by introducing an antagonist. Given the likelihood of developing tolerance to the analgesic activity of opioids with prolonged use, it is often necessary to increase their dose. There are cases, for example, when morphine was administered to patients in doses 35 times higher than its average therapeutic dose in order to increase analgesia. There is a deep intoxication of the body, which can lead to death from respiratory depression. Opioid overdose is a very common cause of death among people with opioid dependence. To make a toxicological diagnosis, it is enough to register three main symptoms – myosis, coma, respiratory depression, requiring urgent administration of antidotes (naloxone, naltrexone).
Opioid analgesics are rarely used in the practice of treating patients with chronic pain of non-oncological origin, including in the population of patients with chronic back pain. Moreover, according to Solomon D.H. et al. The situation has not changed in the last decade. For example, researchers estimated that in 2001, in one state in the United States, only 4% of patients with rheumatoid arthritis received long-term opioid treatment, and less than 1% of patients with chronic low back pain. Over the subsequent 6-year period (in 2006), these figures have not changed dramatically. Moreover, from the wide range of opioid analgesics currently available, doctors preferred the appointment of weak opioids, namely codeine and tramadol (Solomon D.H., 2006). Of course, the widespread use of opioid analgesics in primary clinical practice for the treatment of patients with chronic pain of non-oncological origin is limited, first of all, by doctors’ concern about a wide range of adverse events of opioid pharmacotherapy, the risk of developing tolerance to it and, most importantly, drug (opioid) dependence (Mahowald M.L., Singh J.A., Majeski P., 2005). The problem of “adaptation” of opioid pharmacotherapy in primary clinical practice is unlikely to be resolved in the near future, not only because of their potentially unfavorable safety profile, but also, simply, due to the lack of sufficient, exhaustive evidence of the effectiveness of opioid therapy in patients with chronic non-oncological pain syndromes. Despite the large number of clinical studies conducted to date, in some of which convincing results have been obtained, there is still a lot of debate about the clinical feasibility of opioids in conditions of their “chronic” use by patients with non-oncological pain (Wang H., 2012) (Reinecke H., Sorgatz H., 2009). So, Martell B.A. et al. As part of a meta-analysis of the results of four randomized, (placebo-) controlled trials conducted to compare the effects of opioids, placebo and non-opioid analgesics, there was no significant decrease in pain parameters against the background of chronic opioid use in patients with nonspecific lumbar pain (-0.199, DI95% – 0.49-0.11, p=0.136). Conversely, in five other studies, a direct comparison of the effectiveness of various opioids demonstrated a slight decrease in pain relative to the basal level (-0.93, DI95% – 1.89- -0.03, p=0.055). It should be noted that none of these studies evaluated the effectiveness of analgesia in conditions of opioid use lasting more than 16 weeks (Martell B.A., 2007). At the same time, Mahowald M.L. et al., based on their own extensive clinical experience in the treatment of patients with rheumatic diseases, note that opioid analgesics can be largely effective against this patient population and are associated only with “mild” undesirable effects, and drug dependence is caused only in special cases. In order to substantiate this statement, Mahowald M.L. et al. A retrospective analysis of data obtained from the practice (over 3 years) of therapy of 230 patients who were treated at an orthopedic clinic was performed. A cross-sectional analysis of the effectiveness and safety of opioid therapy was carried out based on the results of interviewing the study subjects. The study involved patients with various orthopedic diseases, including spinal stenosis, intervertebral disc disease, chronic lower back pain after surgery, lumbar spondylosis, vertebral fractures due to osteoporosis, discitis, scoliosis and kyphosis, pain caused by “non-spinal” causes, as well as nonspecific lower back pain. According to the prescription of the attending physician, patients received one of the opioid analgesics: codeine, oxycodone, propoxyphene, morphine, meperidine, hydrocodone, fentanyl or tramadol. In the interview, attention was drawn to the individualized assessment by patients of the effectiveness of pharmacotherapy, the frequency of administration and the types of adverse events. Thus, 152 patients out of 230 patients received opioid analgesics, 94 of them for less than 3 months (short-term) and 58 patients for more than 3 months (long-term). The intensity of the pain syndrome, assessed on a 10-point scale, did not significantly differ in the group depending on the etiology of chronic pain. Opioid therapy was associated with a decrease in pain scores from an average basal value of 8.3±1.5 (before opioid therapy) to 4.5±2.2 (after opioid therapy). Adverse events were observed in 58% of patients, but they were all mild in nature and most often manifested in the form of constipation and sedation. Also, there was no significant increase in drug doses during the opioid intake, which indicates that patients did not develop tolerance to opioid therapy. At the same time, the escalation (increase) of the codeine dose was carried out only in 17 patients who received long-term treatment (more than 3 months), which was due not to the development of tolerance, but to an aggravation of the symptomatic picture of the underlying disease, complications of surgery and other surgical or medical problems. 3 of these patients showed signs of drug dependence (Mahowald M.L., Singh J.A., Majeski P., 2005). Thus, Mahowald M.L. et al. (Mahowald M.L., Singh J.A., Majeski P., 2005) It has been shown that opioid therapy can achieve adequate control of chronic pain in patients with correctly identified causes of pain syndrome. In fact, according to Mahowald M.L. et al., their research data can be used to support specialists conducting pharmacotherapy for chronic non-oncological pain, including back pain, as well as specialists who are reluctant to prescribe opioid analgesics even with appropriate sanctions from national regulatory authorities (Mahowald M.L., Singh J.A., Majeski P., 2005). It is likely that the administration of opioid analgesics may be potentially important for patients with chronic spinal pain to receive the maximum possible benefit from pharmacotherapy in light of the ambiguity of evidence regarding the efficacy/safety of the above-mentioned first-line therapies, i.e. NSAIDs and acetaminophen. Furlan A.D. et al. A meta-analysis of 41 randomized clinical trials involving 6,019 patients was conducted, of which 80% had rheumatoid arthritis, osteoarthritis or spinal pain, 12% had neuropathic pain (postherpetic neuralgia, etc.), 7% had fibromyalgia and 1% had mixed pain. According to the researchers, the methodological quality of the research was 87%. Patients received opioid therapy with mild (tramadol, propoxyphene, codeine) or strong (morphine, oxycodone) analgesics for 1-16 weeks (on average 5 weeks). The rate of early withdrawal of patients from these studies averaged 33% for opioid groups and 38% for placebo groups. Thus, it was shown that opioids significantly more effectively eliminated pain and improved the functional outcomes of pain syndromes of all etiologies in comparison with placebo. Although insignificant, opioids have significantly demonstrated greater effectiveness in comparison with naproxen and nortriptyline in eliminating pain (but not functional activity). Constipation and nausea were significantly more common in the opioid therapy groups. Thus, it was found that weak and strong opioids are superior to placebo in their effect on pain and functional status of patients against all types of chronic back pain. At the same time, other types of drugs had a more positive effect on functional outcomes compared with opioids, and only strong opioids caused reliable pain relief (Furlan A.D., 2006). Khoromi S. and co-author. The efficacy and safety of morphine (15-90 mg), the antidepressant nortriptyline (25-100 mg) and their combination, as well as benztropine as an “active” placebo (at a dose of 0.25-1.0) in the treatment of patients with chronic sciatica (sciatica) were compared. The study included a 4-week period of dose escalation, a 2-week period of therapy at a fixed maximum tolerated dose, and then a 2-week period of gradual dose reduction. Thus, in 28 of the 61 patients who completed the study completely, none of the experimental therapies caused a significant reduction in limb pain and pain ratings. More than 50% of the patients who completed the study reported adverse events associated with taking morphine, nortriptyline or a combination thereof. These results show that nortriptyline, morphine and their combinations may have limited efficacy in the treatment of chronic sciatica (Khoromi S., 2007). Reid M.C. et al. in order to describe the characteristics of elderly patients (G. New York), necessary to predict the outcomes of opioid therapy for chronic non-oncological pain, conducted a retrospective, cohort study involving 133 patients aged an average of 82 years (65-100 years), of which 84% were women, 74 were white patients. The main indications for opioid prescribing were chronic back pain (37%) and osteoarthritis (35%). The average duration of opioid therapy was 388 days (0-1,880 days). In the vast majority of cases, short-acting opioids (hydromorphone, fentanyl, oxycodone, hydrocodone, morphine, propoxyphene) were prescribed, as well as their combinations with acetaminophen. Thus, in 28 of the 61 patients who completed the study completely, none of the experimental therapies caused a significant reduction in limb pain and pain ratings. More than 50% of the patients who completed the study reported adverse events associated with taking morphine, nortriptyline or a combination thereof. These results show that nortriptyline, morphine and their combinations may have limited efficacy in the treatment of chronic sciatica (Khoromi S., 2007). Reid M.C. et al. in order to describe the characteristics of elderly patients (G. New York), necessary to predict the outcomes of opioid therapy for chronic non-oncological pain, conducted a retrospective, cohort study involving 133 patients aged an average of 82 years (65-100 years), of which 84% were women, 74 were white patients. The main indications for opioid prescribing were chronic back pain (37%) and osteoarthritis (35%). The average duration of opioid therapy was 388 days (0-1,880 days). In the vast majority of cases, short-acting opioids (hydromorphone, fentanyl, oxycodone, hydrocodone, morphine, propoxyphene) were prescribed, as well as their combinations with acetaminophen.