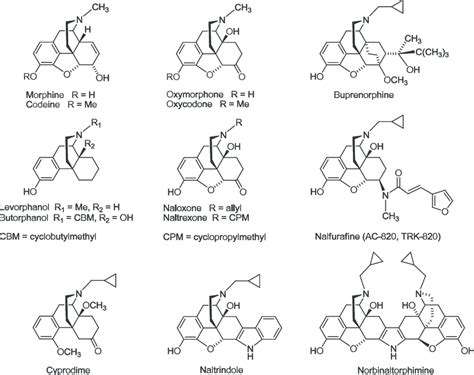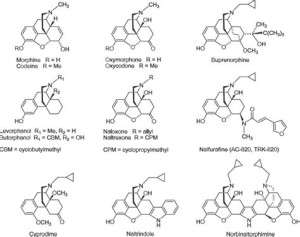
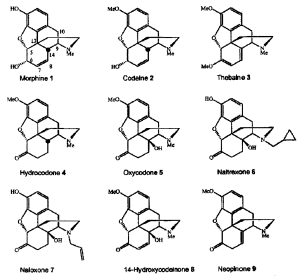
Morphinane is a condensed heterocyclic system (phenanthrenisoquinoline) consisting of a partially hydrogenated (reduced) aromatic core of phenanthrene, some of the cycles of which simultaneously constitute tetrahydroisoquinoline. Morphinane is a condensed heterocyclic system (phenanthrenisoquinoline) consisting of a partially hydrogenated (reduced) aromatic core of phenanthrene, some of the cycles of which simultaneously constitute tetrahydroisoquinoline. Morphinane underlies the chemical structure of opium alkaloids (opiates). Opium is the milky juice of the immature fruits of the sleeping pill poppy, contains the most medically valuable alkaloids, including morphine, codeine, thebaine. They are similar in chemical structure, they are N-methyl derivatives of morphine. In addition to the morphine core, they have a furan cycle.
Codeine is a monomethyl ether of morphine, and ethylmorphine is a monoethyl ether. The codeine content in opium is low (0.2-2%), therefore it is obtained semi-synthetically: by methylation of morphine, and ethylmorphine by ethylation of morphine.
Naltrexone is a semi—synthetic analog of morphine alkaloids, characterized by the presence of a methylcyclopropyl radical in the molecule. Such a change in the chemical structure caused its action as an “antagonist” of morphine. It is used to treat opiate addiction. Naltrexone is synthesized from the opium alkaloid thebaine.
Morphine hydrochloride is prescribed as an analgesic for injuries and other diseases accompanied by pain. The use of morphine causes euphoria, which can lead to morbid addiction and chronic poisoning — morphinism. Codeine in the form of a base and phosphate and ethylmorphine hydrochloride are used as cough suppressants. It should be borne in mind that cases of codeinism from codeine abuse are not uncommon.
Morphine, naltrexone and ethylmorphine are used as hydrochlorides, and codeine is used as a base and phosphate. The formation of salts is due to the presence of a tertiary nitrogen atom, which gives the basic properties to alkaloids and their analogues.
Morphinane derivatives are readily soluble or soluble in water, with the exception of codeine (slow and slightly soluble in water). In ethanol and chloroform, only the codeine base is easily soluble. The rest are slightly or practically insoluble in organic solvents.
Tests of morphine derivatives are based on the use of their reducing and acid-base properties due to the presence in the molecules of the tertiary nitrogen atom, phenolic hydroxyl (morphine), methoxy- (codeine), ethoxy- (ethylmorphine) simple ester groups, as well as acids associated with the organic base (hydrochloric and phosphoric)
Most of the described color reactions are based on the reducing properties of morphine and codeine, which are due to the presence of partially hydrogenated phenanthrenisoquinoline core cycles. In morphine, the reducing properties are expressed to a greater extent due to the presence of phenolic hydroxyl in the molecule.
To identify morphinane derivatives, an apomorphine formation reaction is used, which occurs as a result of exposure to morphine, codeine, ethylmorphine with concentrated sulfuric or hydrochloric acids:
– Concentrated sulfuric acid oxidizes morphine to apomorphine, which then acquires an intense red staining when nitric acid is added.
Morphine, unlike codeine, gives a positive reaction to phenolic hydroxyl – blue staining when interacting with a solution of iron (III) chloride. Morphine hydrochloride with a solution of hydrogen peroxide in the presence of ammonia and 1 drop of copper sulfate acquires a gradually disappearing red staining.
The reducing properties of morphine hydrochloride are manifested when interacting with a solution of potassium iodate. After the addition of diluted sulfuric acid and chloroform, the layer of the latter acquires a pink-purple color due to the iodine formed.
Morphinane derivatives give a positive reaction with the Brand reagent (formaldehyde solution in concentrated sulfuric acid) – red staining, turning into blue-purple. The mechanism of this reaction is different
In the case of morphine, which contains free phenolic hydroxyl in the molecule, a red staining occurs initially (a product of oxidation with sulfuric acid), which quickly turns blue-purple, as an auric dye is formed.
Under the action of concentrated nitric acid, morphine acquires an orange coloration, turning yellow. Codeine and ethylmorphine hydrochloride under these conditions turn an unchanging orange color. Morphine gives a characteristic reaction with a solution of ammonium molybdate in concentrated sulfuric acid (purple staining, turning blue, and then green).
It is also possible to identify the group of alkaloids in question and their synthetic analogues on the basis of the use of precipitation total alkaloid reagents.
A common test for salts of alkaloids and their analogues is the reaction of precipitation of bases from solutions with the addition of ammonia (morphine hydrochloride) or sodium hydroxide solution (codeine phosphate).
The isolated bases have a characteristic melting point. The base of morphine differs from other medicinal substances of this group in that it dissolves in excess of sodium hydroxide due to the presence of phenolic hydroxyl in the molecule.
When ethylmorphine hydrochloride and iodine crystals are heated to a boil in the presence of a sodium hydroxide solution, a characteristic odor of iodoform appears.
Morphine and its derivatives have an absorption spectrum in the UV region characteristic of all substances of this group. Therefore, spectrophotometry is widely used to identify and quantify the absorption maxima of morphine hydrochloride (solvent water or 0.1 M hydrochloric acid solution — at 285 nm, solvent 0.1 M sodium hydroxide solution — at 297 nm), codeine (solvent ethanol — at 284 nm or 0.01 M hydrochloric acid solution — at 285 nm), codeine phosphate (solvent ethanol — at 284 nm and water — at 285 nm), ethylmorphine (solvent water — at 285 nm and ethanol — at 284 nm). The UV spectrum of naltrexone hydrochloride is compared with a standard sample, the maximum absorption is at 280 nm.
The UV spectrum of naltrexone hydrochloride is compared with a standard sample, the maximum absorption is at 280 nm. Hydrochlorides (morphine and ethylmorphine) are titrated with 0.1 M hydrochloric acid solution in anhydrous acetic acid medium after addition of mercury (II) acetate and crystal violet indicator.
The salts of alkaloids and their analogues can also be determined by neutralization with 0.1 M sodium hydroxide solution in an aqueous alcohol medium (phenolphthalein indicator) with the addition of chloroform to extract the released base. There are also known methods for the reverse argentometric determination of morphine hydrochloride (by chloride ion).
For the quantitative determination of naltrexone hydrochloride, the US Pharmacopoeia recommends the HPLC method using a standard naltrexone sample.